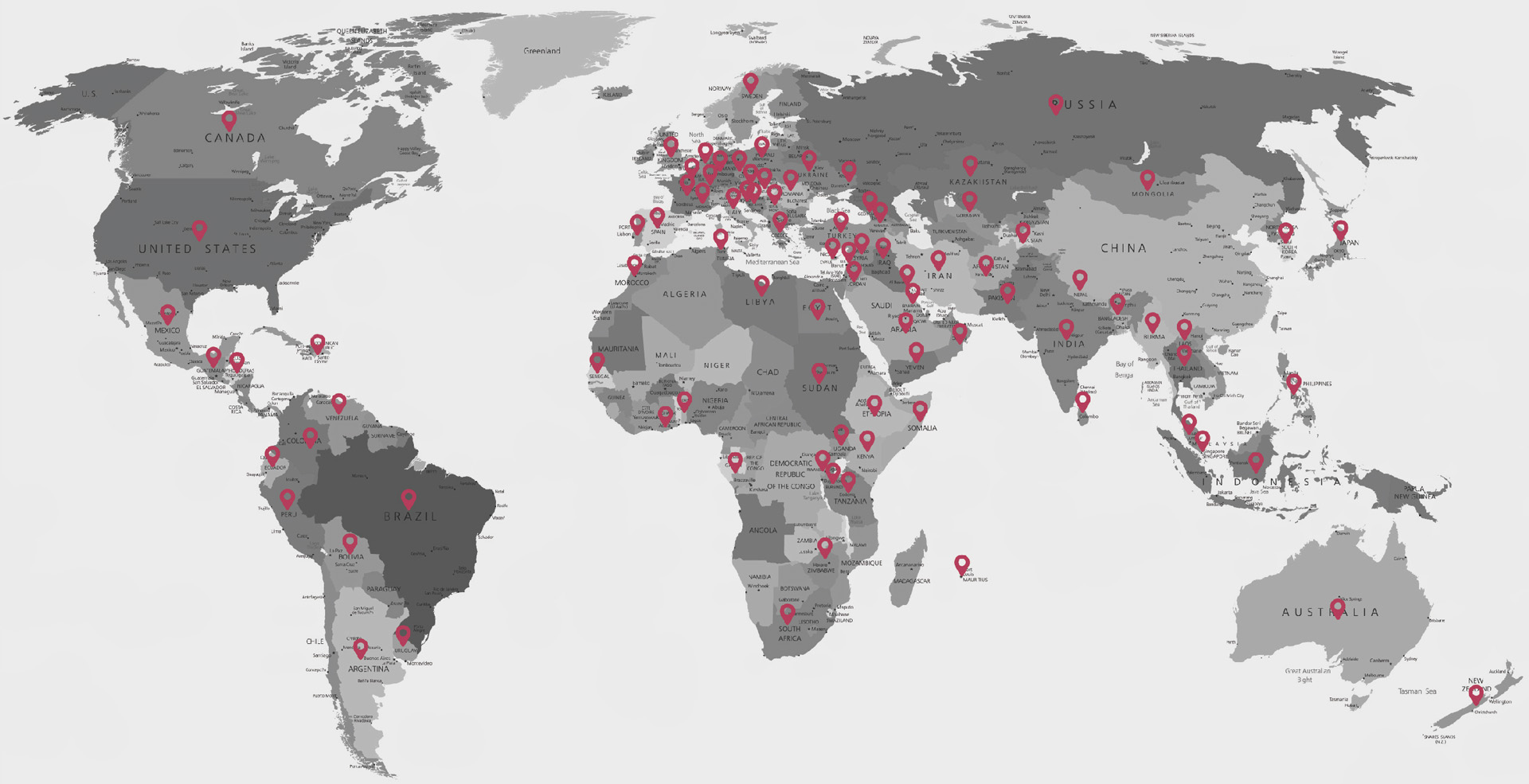
ʻO Yonker (ʻO Xuzhou Yongkang Electronic Science Technology Co., Ltd.) i hoʻokumu ʻia i ka makahiki 2005 a he ʻoihana hana lapaʻau kaulana mākou i ka honua e hoʻohui ana i ka R&D, hana ʻana, kūʻai aku a me ka lawelawe. I kēia manawa, ʻehiku mau lālā o Yonker. ʻO nā huahana ma 3 mau māhele e uhi ana ma mua o 20 mau moʻo e komo pū ana me nā oximeters, nā monitor maʻi, ECG, nā pamu syringe, nā monitor koko, ka oxygen concentrator, nā nebulizers etc., kahi i lawe ʻia aku i nā ʻāina a me nā wahi he 140.
R&D a me ka Hana ʻana
ʻElua mau kikowaena R&D ma Yonker ma Shenzhen a me Xuzhou me kahi hui R&D o kahi o 100 mau kānaka. I kēia manawa, kokoke i 200 mau palapala sila a me nā hōʻailona kālepa i ʻae ʻia. Loaʻa iā Yonker ʻekolu mau kahua hana e uhi ana i kahi ʻāpana o 40000 mika kuea i lako me nā keʻena hoʻokolohua kūʻokoʻa, nā kikowaena hoʻāʻo, nā laina hana SMT akamai ʻoihana, nā hale hana lepo ʻole, nā hale hana hana hoʻoheheʻe pololei a me nā hale hana hoʻoheheʻe injection, e hana ana i kahi ʻōnaehana hana piha a me ka hoʻokele maikaʻi hiki ke hoʻokele ʻia. ʻO ka hopena he kokoke i 12 miliona mau ʻāpana e hoʻokō i nā pono maʻamau o nā mea kūʻai aku honua.
Hui lawelawe ma hope o ke kūʻai aku
Ma lalo o ke alakaʻi ʻana o nā waiwai o "ka ʻoiaʻiʻo, ke aloha, ka pono, a me ke kuleana", loaʻa iā Yonker kahi ʻōnaehana lawelawe kūʻokoʻa ma hope o ke kūʻai aku no ka hāʻawi ʻana, OEM a me nā mea kūʻai hope. ʻO nā hui lawelawe pūnaewele a me waho pūnaewele ke kuleana no ke ola holoʻokoʻa o ka huahana. I mea e hoʻomaikaʻi ai i ka pono o ka lawelawe, nā hui kūʻai aku a me nā lawelawe ʻo Yoner ma 96 mau ʻāina a me nā ʻāpana, i loko o 5 mau hola e pane i ka ʻōnaehana hoʻopili koi, e hāʻawi i nā mea kūʻai aku me ke kākoʻo loea ʻoi aku ka ʻoihana.
Hoʻokele Kūlana a me ka Palapala Hōʻoia
ʻOi aku ka maikaʻi o ka ʻōnaehana nānā ʻana i ka maikaʻi o ke kaʻina hana holoʻokoʻa o Yonker no ka hoʻolālā honua o ka brand Yonker. A hiki i kēia manawa, ʻoi aku ma mua o 100 mau huahana i loaʻa iā CE, FDA, CFDA, ANVISN, TUV ISO13485, CMD ISO9001 a me nā palapala hōʻoia ʻē aʻe. Uhi ka nānā ʻana i nā huahana iā IQC, IPQC, OQC, FQC, MES, QCC a me nā kaʻina hana hoʻomalu maʻamau ʻē aʻe, ua helu ʻia ʻo Yonker ma ke ʻano he National High-tech Enterprise, National Intellectual Property Advantage Enterprise, Jiangsu Medical Device Manufacturing Enterprise Member Unit. A ua mālama ʻo Yonker i nā pilina hana lōʻihi me Renhe Hospital, Respironics, Philips, Suntech Medical, Nellcor, Masimo a me nā hōʻailona kaulana ʻē aʻe.
ʻIke o ka ʻoihana
E hoʻoikaika i ke kumu o ke ola a me ke olakino
Nā mea lapaʻau 100 kiʻekiʻe loa o Kina 2025
Nā waiwai koʻikoʻi o ka ʻoihana:ʻOiaʻiʻo, aloha, pono a me ke kuleana
Misiona a ka ʻoihana:E hoʻopaʻa mau i ka hāʻawi ʻana i nā mea kūʻai aku i nā huahana maikaʻi me nā kumukūʻai kiʻekiʻe a hoʻoneʻe i nā naʻau o kānaka
Ua hōʻike ʻo Periodmed, ka lālā o Yonker Group, i nā huahana lapaʻau hou loa ma ka 2024 Shanghai CMEF












Ua hoʻomaka mua ʻo Periodmed Medical, ka lālā o Yonker Group, ma ka 2024 Dubai Arab Health Exhibition.












Hōʻikeʻike Halemai a me nā Lako Lapaʻau ʻo Düsseldorf International ma Kelemania








2023 Kina (Shenzhen) 88th Kina International Medical Equipment (Autumn) Expo
-2.png)
-15.jpg)
-71.jpg)
-2.jpg)
-37.jpg)
-14.jpg)
-24.jpg)
-13.jpg)
-35.jpg)
-33.jpg)
-29.jpg)
-21.jpg)
-32.jpg)
-16.jpg)
-6.jpg)
-39.jpg)
Hale Hōʻikeʻike Lapaʻau ʻo Yonker ma Jakarta, Indonesia ma ka Hale B 238 a me 239








Nā Huahana a Yonkermed i Hōʻike ʻia ma ka Hōʻikeʻike Ola Kino o ʻApelika Hema 2023




Hōʻikeʻike Mea Lapaʻau Yonker Hou 2023








Hui Elite








Hoʻohanohano ʻoihana ʻoihana
Ua helu ʻia ʻo Yonker ma ke ʻano he National High-tech Enterprise, National Intellectual Property Advantage Enterprise, Jiangsu Medical Device Manufacturing Enterprise Member Unit. A ua mālama ʻo Yonker i nā pilina lōʻihi me Renhe Hospital, Respironics, Philips, Suntech Medical, Nellcor, Masimo a me nā hōʻailona kaulana ʻē aʻe.
A hiki i kēia manawa, ʻoi aku ma mua o 100 mau huahana i loaʻa iā CE, FDA, CFDA, ANVISN, TUV ISO13485, CMD ISO9001 a me nā palapala hōʻoia ʻē aʻe. Uhi ka nānā ʻana i ka huahana iā IQC, IPQC, OQC, FQC, MES, QCC a me nā kaʻina hana hoʻomalu maʻamau ʻē aʻe.







Nā Respironics Etco2

ʻO ka mahele kukui Philips

Mea hoʻolako modula koko honua

45% ka māhele mākeke o ka SPO2 honua


